Peer-review started: June 29, 2015
First decision: November 6, 2015
Revised: November 24, 2015
Accepted: December 13, 2015
Article in press: January 4, 2016
Published online: March 27, 2016
Processing time: 274 Days and 11.9 Hours
The recombinant DNA technology enabled the production of a variety of human therapeutic proteins. Accumulated clinical experience, however, indicates that the formation of antibodies against such proteins is a general phenomenon rather than an exception. The immunogenicity of therapeutic proteins results in inefficient therapy and in the development of undesired, sometimes life-threatening, side reactions. The human proteins, designed for clinical application, usually have the same amino acid sequence as their native prototypes and it is not yet fully clear what the reasons for their immunogenicity are. In previous studies we have demonstrated for the first time that interferon-β (IFN-β) pharmaceuticals, used for treatment of patients with multiple sclerosis, do contain advanced glycation end products (AGEs) that contribute to IFN-β immunogenicity. AGEs are the final products of a chemical reaction known as the Maillard reaction or glycation, which implication in protein drugs’ immunogenicity has been overlooked so far. Therefore, the aim of the present article is to provide a comprehensive overview on the Maillard reaction with emphasis on experimental data and theoretical consideration telling us why the Maillard reaction warrants special attention in the context of the well-documented protein drugs’ immunogenicity.
Core tip: The Maillard reaction occurs spontaneously in host cells and causes covalent modifications, proteolysis and crosslinking of therapeutic proteins. These are gross structural changes, which upon administration may provoke in patients classical type innate and adaptive immune responses. The consequences of the Maillard reaction, however, reach far beyond. Specific and non-specific cellular receptors for the advanced products of the Maillard reaction may further enhance the immune response and elicit inflammation. All together the Maillard reaction actions are expected to result in drug neutralization and side effects in treated patients such as inflammatory and hypersensitivity reactions.
- Citation: Tsekovska R, Sredovska-Bozhinov A, Niwa T, Ivanov I, Mironova R. Maillard reaction and immunogenicity of protein therapeutics. World J Immunol 2016; 6(1): 19-38
- URL: https://www.wjgnet.com/2219-2824/full/v6/i1/19.htm
- DOI: https://dx.doi.org/10.5411/wji.v6.i1.19
Protein pharmaceuticals are the major group of biopharmaceuticals used today in medicine for treatment of a large number of diseases. Accumulated clinical experience indicates that protein drugs can elicit an immune response especially when applied in multiple doses over a prolonged period. The antibodies (Abs) in treated patients are produced either by a classical immune response against non-self-antigens or by distortion of the immune tolerance[1]. The development of anti-drug Abs is a slow process, in which the frequency of occurrence and the titer of the antibodies vary widely from low levels in most cases to very high, for example during treatment with some interferon-β pharmaceuticals (IFN-β)[2]. The anti-drug Abs may have no clinical consequences[3-5]. However, some studies show that Abs may neutralize drug activity and that of the endogenous drug counterparts thus provoking severe complications in patients[6-8]. The most frequent side effects are flu-like symptoms and injection-site reactions[9,10], although cases of allergy, anaphylaxis and serum sickness have also been reported[11-16].
The amino acid sequence of therapeutic proteins may differ from that of the native human prototypes and such proteins are therefore recognized by the immune system as non-self. This was the case with porcine insulin used nearly 60 years (from 1920 to 1980) for treatment of diabetes. Porcine insulin differs by only one amino acid from human[17] but all patients treated with porcine insulin developed anti-insulin Abs[18]. To compensate for drug neutralization in such cases drug dosages are increased thus further boosting the immune response. The final result of such a vicious cycle is loss of therapeutic efficacy[19,20]. The deviation from the native protein structure may explain why insulin and other therapeutic proteins of animal origin are immunogenic. However, the production of human insulin by the recombinant DNA technology did not fully solve the problem with the immunogenicity. Interestingly, the deletion of Tyr-19 in human insulin resulted in reduced immunogenicity[21]. We suppose that this Tyr-residue might be involved in covalent aggregation of insulin via the formation of dityrosine crosslinks.
To date, the reasons for the immunogenicity of human proteins are not fully understood. The dose and duration of treatment, the route of drug administration and patients’ inborn characteristics may modulate the immune response[22]. Excipient substances, used in drugs to stabilize proteins, may also be of particular relevance[23-25]. However, the reasons for immunogenicity are mainly attributed to protein structural changes occurring during fermentation, purification, drug formulation and storage, including amino acid substitutions, non-native (or lack of) glycosylation, proteolysis, aggregation (both covalent and non-covalent), denaturation, deamination and oxidation. Some of these structural alterations (covalent aggregation and proteolysis) may occur during the Maillard reaction, which relation to drugs’ immunogenicity is poorly studied and has inspired us to write the current review.
At the beginning of the past century the French chemist Louis Camille Maillard conducted research on peptide synthesis[26]. At that time, when the mechanisms of protein biosynthesis were still a mystery, his far reaching goal was to understand the natural way of amino acid polymerization under mild physiological conditions. For this purpose Maillard used D-glucose, a widespread sugar in biological systems, as a soft condensing agent. Thus he realized that glucose, through its aldehyde group, is capable of reacting with amino acids[26]. In this way Maillard did not only make a contribution to basic organic chemistry but ingeniously predicted that “The consequences of these facts appear… interesting in various fields of science: Not only in human physiology and pathology’’[26].
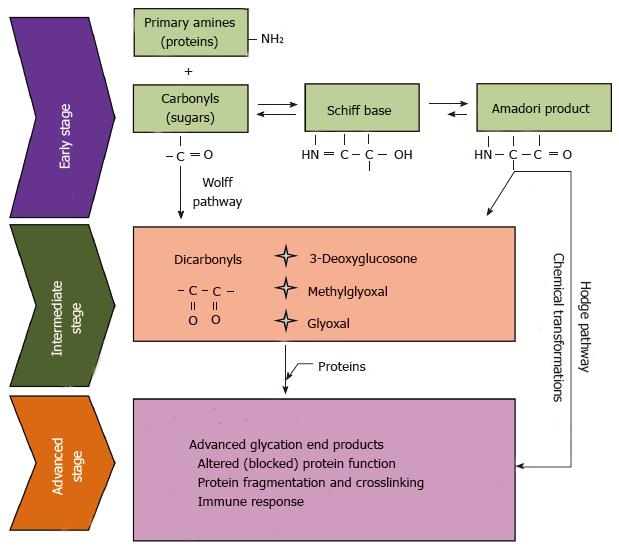
The significance of the Maillard reaction, known also as non-enzymatic browning reaction or carbonyl-amine reaction, for food chemistry was recognized soon. However, it took many years for biologists and physicians to grasp the physiological role of the Maillard reaction. Studies in the late 1960s revealed the existence of an abnormal fast-moving hemoglobin band in diabetic patients during routine electrophoretic screening for hemoglobin variants[27,28]. In the same year, it has been shown that the fast-moving hemoglobin subfraction HbA1c can be prepared in vitro by incubation of hemoglobin with glucose in the absence of enzyme catalysts[29]. Later on, in the scientific lexicon were introduced the terms “non-enzymatic glycosylation” and “glycation” in order to distinguish the Maillard reaction, proceeding in biological systems, from the enzymatic glycosylation, which is a quite different, genetically programmed process. In the early 1980s, it has been hypothesized that glycation plays an important role in the pathogenesis of diabetic complications and aging[30,31]. Subsequently, this hypothesis found a plethora of experimental support, and many reviews have been dedicated to the link between glycation, diabetes and aging[32-38]. Although the carbonyl-amine reaction was discovered a century ago, its chemistry remains a still growing avenue of research. Two well defined stages can be distinguished in the Maillard reaction - early and advanced. The early stage includes reversible formation of Schiff bases between carbonyl and amino groups of the reactants, followed by a rearrangement of the Schiff bases to significantly more stable aldoamines (Amadori products) or ketamine (Heyns products) (Figure 1).
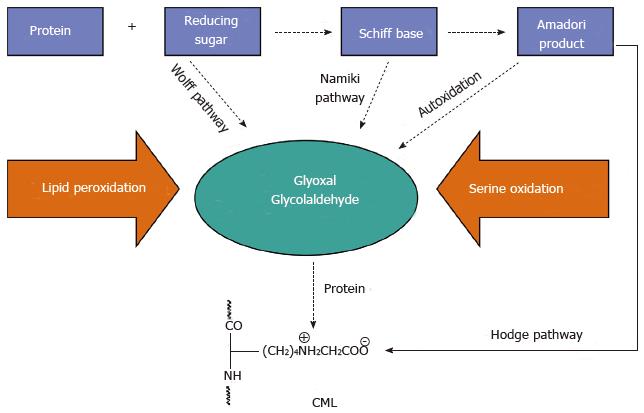
The early stage of the Maillard reaction is relatively well understood[39,40]. However, the same does not hold true for the chemical transformations of the Amadori and Heyns products into the so called advanced glycation end products (AGEs)[41]. Generally, these are dehydrogenation, dehydration, cyclization, condensation, isomerization, oxidation, and fragmentation reactions, taking place in thе advanced stage of Maillard reaction. Under anaerobic conditions, AGEs are derived directly from the Amadori product. However, under aerobic conditions, oxidative degradation of the Amadori products takes place, leading to the generation of highly reactive dicarbonyl compounds such as glyoxal (G), methylglyoxal (MG) and 3-deoxyglucosone (3DG)[42,43]. This “interruption” between the early and the advanced stage is what we call the intermediate step of Maillard reaction. In turn, the intermediate products, which are small, diffusible and highly reactive species, again attack amino compounds, thus propagating the initial chemical burden. The formation of AGEs, either directly from the Amadori product or through its degradation intermediates, is known as the classical, or Hodge pathway[40]. The Schiff base, formed early in the Maillard reaction, may also undergo non-enzymatic fragmentation to α-oxoaldehydes, which initiate another chain of chemical transformations known as the Namiki pathway[44]. In addition, under physiological conditions free monosaccharides undergo a transition metal ions catalyzed autoxidation to H2О2 and the corresponding ketoaldehydes, which are precursors for AGEs formation in the Wolff pathway, also recognized as a separate reaction chain in the Maillard chemistry[45-47]. The picture becomes increasingly colorful, pasting therein the carbonyl products released during lipid peroxidation, which also react with amines to form AGEs-like structures called Advanced Lipoxidation End Products (ALEs)[48,49]. AGEs/ALEs are covalent adducts with diverse stability and physicochemical characteristics such as blue fluorescence, brown color and crosslinking properties. When accumulating in biological amines such as proteins, DNA and amino-lipids, AGEs may significantly impair their structure and physiological function. The chemical structure of a number of AGEs formed in vitro as well as in human plasma and tissues has been determined so far[50-57].
Nε-(carboxymethyl)lysine
Nε-(carboxymethyl)lysine (CML) is one of the best-characterized AGEs[58]. It is formed in the advanced stage of the Maillard reaction and always accompanies the formation of brown and fluorescent AGEs, although per se it is colorless and non-fluorescent. If proteins are glycated with either ribose or 3DG, then CML is formed only when the reaction is carried out under aerobic conditions[52,59]. This is why the detection of CML is an indication that protein modifications have occurred in the presence of oxygen. CML is formed by different oxidation mechanisms in vitro, involving not only reducing sugars. It may also be a product of lipid peroxidation[48] and serine oxidation[60] (Figure 2), and because of that CML should not be considered a typical glycation marker. In most cases of in vitro glycation CML is the main product found in proteins[61], and is also a dominant AGEs antigen in tissue proteins[62]. Direct formation of CML from the Amadori product through its autoxidation has been reported by Miki Hayashi et al[63]. The authors used as a model of Amadori compound glycated human serum albumin (HSA) and found that CML is formed by heat treatment of glycated HSA over 80 °C in a time-dependent manner. Further rise in the temperature to 100 °C resulted in the formation of the glycation intermediates G, MG and 3DG. The formation of CML in HSA was inhibited in the presence of a reducing agent (sodium borohydride), a chelator of transition metal ions (diethylenetriamine pentaacetic acid), or a trapping reagent for a-oxoaldehydes (aminoguanidine), which indicates that CML (AGEs) accumulation in proteins is manageable.
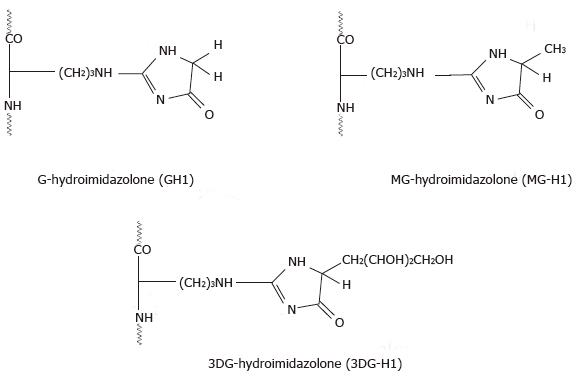
Hydroimidazolones are formed in a reaction of the guanidino group of arginine with G, MG and 3DG[64-66]. The resulting products undergo structural and stereochemical isomerization, which were extensively studied by Ahmed et al[54,65]. The authors found that hydroimidazolones formed in the reaction of arginine with methylglyoxal (MG-H) are composed of three structural isomers designated as MG-H1, MG-H2 and MG-H3. The reaction of arginine with G and 3DG yielded similar structural isomers (Figure 3)[67]. Due to racemization in the hydroimidazolone ring, each structural isomer may exist in two epimiric forms, which cannot be resolved without prior derivatization. As a rule, the stability of hydroimidazolones decreases with increasing pH and each structural isomer has a distinctive stability and half-life. The half-life of the individual stereoisomers of MG-H increases in the following order: MG-H1 > MG-H2 >> MG-H3. Under physiological conditions (pH 7.4, 37 °C) the half-life of MG-H1 is approximately 12 d. Ahmed et al[65] suggested that in contrast to CML, hydroimidazolones are not accumulating in long-lived proteins over time in vivo but rather reflecting short episodes of enhanced protein glycation under conditions of abnormal rise in the concentration of α-oxoaldehydes. The authors also assumed that Nω-(1-carboxyethyl)arginine and Nω-(1-carboxymethyl)arginine, which are more stable and found in in vivo glycated proteins, are probable degradation products of hydroimidazolones.
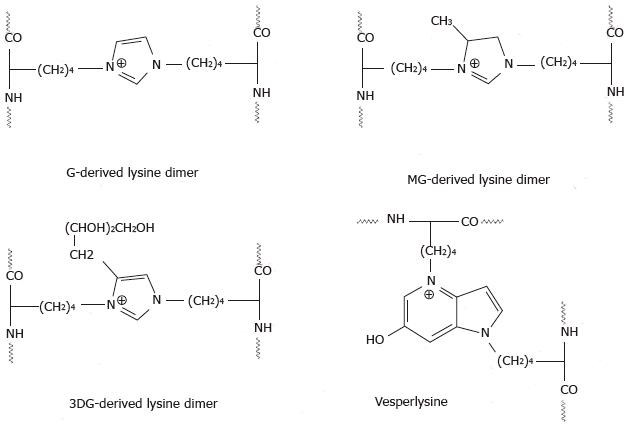
The Maillard reaction not only yields sugar adducts on proteins but also results in gross structural changes such as proteolysis[68-70], and intra- or inter-molecular crosslinking[71,72]. Proteolysis of glycated proteins usually occurs at lysine and arginine residues[73] but may also happen randomly as a result of transition metal ions catalyzed oxidation[68,69]. In contrast, covalent crosslinking of polypeptide chains involves exclusively lysine and arginine residues. Lysine residues on the same or on different polypeptide chains become involved in the formation of shared AGEs structures such as G-derived lysine dimer (GOLD)[74], MG-derived lysine dimer (MOLD)[75], 3DG-derived lysine dimer (DOLD)[76], G-derived lysine-lysine crosslinks GOLA and GALA[77,78], glucose lysine dimer GLUCOLD[79], crossline[80,81] and vesperlysines[82] (Figure 4)[67]. AGEs crosslinks formed between lysine and arginine residues are G-derived arginine-lysine dimer (GODIC), MG-derived arginine-lysine dimer (MODIC)[83], 3DG-derived arginine-lysine dimer (DOGDIC)[84], pentosidine[78] and glucosepane[84]. To the best of our knowledge, to date crosslinks formed between two arginine residues or between arginine/lysine residues and other amino acids have not been detected neither in vivo nor in in vitro glycation reaction, although the possible involvement of the cysteine thiol group in the formation of such crosslinks has been proposed[85].
Early and advanced glycation products are formed in the human body throughout its existence. Many glycation adducts, appearing on proteins in model reactions in vitro, have been detected also in physiological systems. Fructoselysine (FL), the Amadori product formed by glucose on the ε-amino group of lysine, has a relatively short half-live (2-6 wk)[86]. As a rule, early glycation in healthy subjects is not age-dependent, although some rise in the physiological FL concentration has been observed during aging. For example, the concentration of the collagen-bound FL increases from 5 mmol/mol Lys in 20-year-old individuals to 7 mmol/mol Lys in 70-year-old subjects[87]. Short-lived proteins such as albumin (half-life ca. 20 d) and even hemoglobin (half-life ca. 120 d) accumulate in vivo predominantly early glycation products. Approximately 10% of HSA of healthy subjects is modified by FL at Lys-525[88], and incubation of HSA with glucose under physiological conditions in vitro leads to glycation of the same Lys residue[89]. Other Lys residues in HSA including Lys-439, Lys-199 and Lys-281 are also involved in FL formation in vivo[90] and it has been reported that glycation impairs HSA binding to physiologically important ligands such as bilirubin and cis-parinaric acid[89]. The historically crucial hemoglobin HbA1c contains glucose-derived Amadori product at the amino group of the hemoglobin β-chain N-terminal valine (βVal-1) and amounts 4%-6% of the total hemoglobin in healthy individuals. Except the N-terminal valine, some internal Lys residues in HbA1c also become glycated[91]. Under hyperglycemia HbA1c levels increase several times and correlate positively with the chronic diabetic pathology[92].
Most AGEs are either not formed at physiological glucose concentrations (5 mmol/L) or their levels are quite lower (ten to thundered times) compared with hyperglycemic conditions. This makes AGEs relevant biomarkers of the glycation status of individuals, which changes with age and in disease state such as diabetes and kidney failure. Because of their great diversity, AGEs cover a wide concentration range in vivo (0.001 to 15 mmol/mol modified amino acid)[86]. Physiologically important AGEs are those having long half-life (CML, CEL and pentosidine) and/or high concentration (hydromidazolones). Stable AGEs accumulate on long-lived proteins such as skin and cartilage collagen[93-96], and lens proteins[97], although CML has also been detected in the short-lived HSA of diabetic patients[98]. Hidroimidazolones, albeit relatively unstable, may also appear on long-lived proteins such as human lens proteins[99].
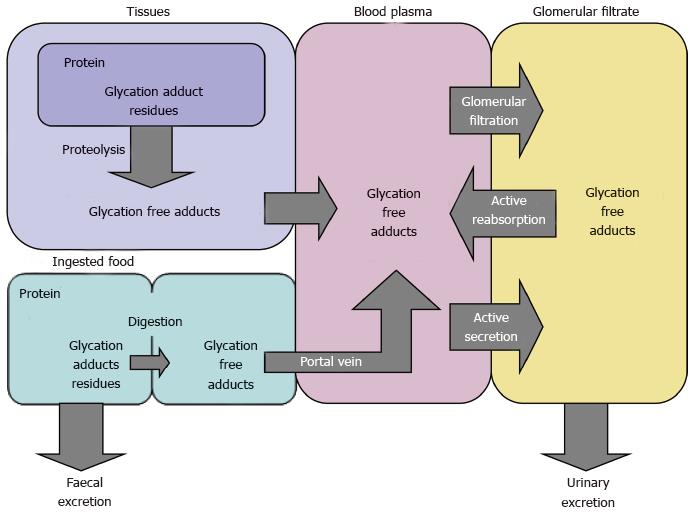
Maillard reaction products (MRPs), which are found in the body, are classified into three groups - MRPs in proteins (> 12 kDa), MRPs in peptides (< 12 kDa) and free MRPs in amino acids. The term MRPs is more general than AGEs and applies to both early glycation products and AGEs. Peptide MRPs are detected mainly in the portal venous plasma and in the urine, and are most likely degradation products of glycated proteins. Free MRPs are formed during proteasome/lysosome degradation of glycated cellular proteins, then released into the bloodstream and excreted in the urine, which ensures efficient functioning of the cell proteome[100,101]. Free MRPs may appear in vivo also as a result of amino acids glycation though this latter source of free MRPs is disputable[55].
The impact of glycation on human physiology depends on many factors including the target amino compound and its half-life, the adduct stability and its location. Especially harmful are the consequences of the formation of covalent cross-links in proteins that render them resistant to proteolysis[102]. Both early glycation products and AGEs can cause damage via various mechanisms such as (1) release of oxygen radicals by early products[103]; (2) altered (blocked) activity of enzymes[104,105], receptors[106] and regulatory proteins[107,108]; (3) crosslinking of structural proteins[78,109]; (4) damage of signaling pathways[110,111], (5) damage of protein recycling[112,113]; and (6) induction of an immune response[114].
Sugar-rich and processed foods are also a source of FL and AGEs in the human body[115]. The biodistribution and metabolism of these products have not been systematically studied, except the dietary intake of FL in humans[116]. Among foods rich in FL and AGEs are bread, biscuits, chocolate, breakfast cereals and hot milk. It has been reported that lactulose-lysine, an Amadori product formed during heat treatment of milk, is poorly digested in the gastrointestinal tract[117]. Some microorganisms can hydrolyze FL in the intestine. For example, an enzyme has been discovered in E. coli that degrades Nε-fructoselysine-6-phosphate to lysine and glucose-6-phosphate[118]. FL ingested with food and resorbed by the intestine, enters the circulatory system and then penetrates liver and muscle cells by passive diffusion. It is not clear yet whether lysine and glucose can be recycled from FL, because no human enzymes have been identified so far catalyzing the breakdown of FL to free lysine and glucose. In rats, about 60% of dietary FL is excreted in urine[119], whereas in humans this percentage is only 3%. In newborns, however, 16% of FL is found in the urine, and 55% is recovered in the feces[116] (Figure 5).
Foods with high FL and AGEs content are poorly digestible, because glycated proteins are resistant to proteolysis. In addition, some AGEs inhibit intestinal proteases[120]. It is thought that dietary AGEs are absorbed mainly in the form of free and peptide MRPs. The highest concentration of absorbed food AGEs is expected in the plasma of the portal vein, where MG-H1 is detectable mainly in peptides[121]. Although CML is formed in vivo, it is hypothesized that urinary CML is mostly of exogenous nature[122]. Studies with rats have shown that another product of the advanced glycation, 5-Hydroxymethyl-2-furaldehyde (HMF), administered per os or intravenously is present in the liver but is mostly in the kidney and the bladder. Also, HMF or its metabolites are rapidly eliminated in the urine with a recovery of 95%-100% after 24 h[123]. Only 10% of dietary AGEs are absorbed, of which only 30% are excreted in the urine of healthy individuals with unimpaired renal function[124]. Some dietary AGEs, after entering blood circulation, interact with low density lipoprotein (LDL) and tissue proteins (e.g., collagen) or bind to cellular receptors for AGEs to trigger intracellular oxidative stress, endocytosis and degradation of AGEs.
Many cellular receptors are capable of binding AGEs. The AGE-receptor complex consists of AGE-R1 (oligosaccharyl transferase-48), AGE-R2 (80K-H phosphoprotein)[125,126] and AGE-R3 (galectin-3)[127]. Some receptors belonging to different classes of the scavenger receptor (SR) supergroup[128,129] also bind AGEs. These are SR-A1 (SCARA1)[130] and SR-A1.1 (SR-AII)[131], SR-B1 (SR-BI)[132] and SR-B2 (CD36)[133], SR-E1 (LOX-1)[134], SR-H1 (FEEL-1) and SR-H2 (FEEL-2)[135]. These receptors are involved in detoxification of AGEs by intracellular degradation (endocytosis). Best characterized is the specific receptor for AGEs called Receptor for Advanced Glycation End Products (RAGE)[136]. RAGE is a multi-ligand receptor of the immunoglobulin superfamily, which plays a key role in inflammatory responses. Its extracellular domain is composed of three immunoglobulin-like domains - one V (variable) type Ig domain followed by two C (constant) type Ig domains[137]. RAGE also possesses a transmembrane domain and a cytoplasmic tail of 43 amino acids. Crucial for ligand binding is the V-domain, while the cytoplasmic tail is implicated in further transduction of captured signals[138]. RAGE is expressed weakly on a number of cell types and tissues under physiological conditions, however, an increased expression is observed in sites of ligands’ deposition. RAGE exists also in a soluble form (sRAGE)[139], which is hypothesized to participate in detoxification of circulating ligands.
Apart from AGEs, ligands for RAGE are pro-inflammatory cytokine-like mediators from the S100/calgranulin family[140], the β-amyloid peptide[141] and amfoterin also known as High Mobility Group Box 1 (HMGB1) - a nuclear protein released during cell necrosis[142-144]. AGEs and non-AGEs ligands bind to RAGE on endothelial cells, neurons, smooth muscle and immune cells to activate a variety of signaling pathways including expression of NF-κB - a transcription factor, which plays a key role in regulation of the immune response[145]. While expressed inducibly on T-cells of healthy subjects upon T-cell receptor activation, RAGE is constitutively synthesized in diabetics’ T-cells, and a role for RAGE in the adaptive immunity has been proposed[146,147]. In has been demonstrated that ovalbumin (OVA) modified with AGEs (pyrraline), but not native OVA, induces SR-A mediated uptake of the antigen by dendritic cells and enhances CD4+ T-cell immunogenicity and potential antigenicity of OVA[148,149]. Characteristic for RAGE is that it recognizes tertiary structures rather than amino acid sequences. This feature endows RAGE with properties of the pattern recognition receptor (PRR), which recognizes repeated antigenic motifs of the type of pathogen-associated molecular patterns (PAMPs)[150]. RAGE can also recruit immune cells in the sites of inflammation. For example, RAGE on endothelial cells can function as an adhesive receptor interacting with the leukocyte β2-integrins[151]. Intriguing findings in recent years suggest that RAGE’s actions contribute to perpetuation of AGEs production by sustaining oxidative stress and inflammation, and by suppressing the detoxification of MG as one of the major AGEs precursors[152,153].
Apart from cellular immune responses, AGEs may elicit a humoral immunity. Excessive accumulation of AGEs with age and in pathology appears to correlate with elevated levels of anti-AGEs antibodies[154]. Glycated proteins [histone H2A[155], HSA[156], poly-L-lysine (PLL)[157], IgG[158]], DNA[159,160] and LDL[161,162] have demonstrated higher immunogenicity in experimental animals than the non-glycated counterparts, and sera of diabetic patients were found to exhibit higher binding activity to glycated HSA[163], PLL[157], IgG[158] and DNA[159] than to the unmodified molecules. In addition, patients with renal failure of diabetic or non-diabetic etiology had higher autoantibody activity against CML (an AGE structure) than normal subjects or diabetics without renal failure[164]. The injection of rats with in vitro glycated rat skin collagen leads to the formation of anti-collagen Abs, which do not cross-react with non-glycated collagen. The binding of the serum Abs to the glycated collagen is inhibited by 92% when the reaction is conducted in presence of glycated Lys as a competitor. This result indicates that glycated Lys residues on collagen are the most probable epitopes captured by the anti-collagen Abs[165]. Of note, sera from streptozotocin-induced diabetic rats contained Abs that clearly bound glycated collagen.
Vay et al[166] have observed 289 diabetic patients and 120 healthy individuals for serum Abs against CML (anti-CML Abs). Although they have found an increased titer of anti-CML Abs (IgG-isotype) in sera of diabetic patients compared to controls (P < 0.0001), there was no correlation between the titer of the anti-CML Abs and patients’ glycemic status. In a similar study including 58 children with type I diabetes, 19 children have been found to be anti-AGEs Abs positive[167]. In contrast to the previous study, the authors have found that the titer of the anti-AGEs Abs correlates positively with some diabetic markers such as HbA1c, microalbuminuria and retinopathy. While some studies show higher titer of circulating anti-AGEs Abs in diabetic patients than in healthy individuals[164,166], others report on the reverse condition, i.e., lower anti-AGEs Abs titers in diabetics than in normal controls[168,169]. To explain this paradox the authors suggested that circulatory anti-AGEs Abs are either captured by tissue-bound AGEs[168] or entrapped in immune complexes[169] hampering their determination. If real, such events could explain the lack of a correlation between the titer of the serum anti-CML Abs and the patients’ glycemic status in the above cited study[166].
It is well known that some patients with rheumatoid arthritis (RA) develop auto-Abs against the IgG constant (Fc) region, which is designated as rheumatoid factor (RF). A pilot study with RA patients has shown that only RF-positive patients have serum IgM Abs binding to in vitro glycated IgG (IgG-AGEs), suggesting that the IgM anti-IgG-AGEs together with IgG-AGEs may contribute to the pathogenesis of RA[170]. Further studies have shown that immune complexes isolated from patients’ sera indeed contain AGEs (CML and imidazolone) modified IgG. It is very likely that the anti-IgG-AGEs prevent the normal clearance of IgG-AGEs by AGEs receptors[171]. Noteworthy, as with most biologics, some RA patients treated with antagonists of the human tumor necrosis factor (TNF) (infliximab, etanercept and adalimumab) develop anti-drug antibodies[172]. The TNF-antagonists are in fact anti-TNF Abs of IgG isotype[172] and in light of the current review two events could be proposed: (1) if the Fc regions of the therapeutic anti-TNF Abs are also glycated (anti-TNF-AGEs Abs), they could compete with patients’ IgG-AGEs for binding RF in human plasma; and (2) if the patients’ antibodies against the glycated biologic are specifically directed against the glycated moiety (AGEs) of the anti-TNF-AGEs Abs, apart from binding the drug, they would be also capable of interacting with patients’ IgG-AGEs. The net result of this hypothetical and intertwined scenario is difficult to predict, but it should be taken into consideration by pharmacists, pharmacologists and clinicians. Intriguingly, subcutaneous immunization with AGEs modified LDL (AGEs-LDL) significantly inhibited atherosclerosis progression in hyperlipidemic diabetic mice possibly through activation of specific humoral and cell mediated immune responses[173]. The anti-atherogenic effect of the anti-AGEs-LDL Abs, however, is disputable yet[174] in so far as immune complexes containing such Abs are detected in human sera[175] and shown to be important predictors of carotid intima-medial thickening in patients with type 1 diabetes[176].
The most important structural change contributing to protein drugs’ immunogenicity is aggregation, which may be both covalent and non-covalent. The non-covalent aggregation results from interaction between non-native transient protein conformers with partially preserved secondary structure called aggregation competent particles. Such particles accumulate progressively in the time course of protein storage due to structural fluctuations and beget intermolecular interactions resulting in protein aggregation. The aggregation process is often accompanied by protein precipitation and loss of biological activity[177]. It is believed that the formation of aggregates proceeds by a cooperative mechanism, i.e., the non-native conformers react with other native molecules to render them aggregation competent. The formation of dimers and higher order multimers in turn accelerates the aggregation process[178,179].
Aggregates in protein drugs substantially contribute to immune response in treated patients[23,180-185]. Studies with interferon-α have shown that the magnitude of the immune response does not depend on the size of the aggregates but rather on the structure of the protein molecules involved in aggregation. Aggregates, formed by molecules with largely preserved secondary structure (native-like) are more immunogenic compared to aggregates composed of fully denatured molecules[22,186]. This could explain why Purohit et al[187] have not observed an immune response against recombinant human factor VIII in model animals. Before injection into animals the authors heated the protein to stimulate aggregation, which perhaps caused its denaturing. The prevalent hypothesis, which explains the immunogenicity of the protein aggregates, is the “array” hypothesis[188-194]. The human immune system is specialized to recognize proteins that are presented in an array format, as is the case with viral capsids and bacterial cell walls. In fact, aggregated proteins are arranged in similar “pathogen-like” structures and thus may be sensed by the immune system as foreign antigens.
The enzymatic glycosylation is an inborn post-translational modification of many eukaryotic proteins and in most cases is indispensable for their biological activity and stability. Also, the lack of native polysaccharide residues may unmask potentially immunogenic epitopes in proteins. Increasing evidence in the last decade shows that glycoproteins are not a eukaryotic privilege. Competent to enzymatically glycosylate proteins are many prokaryotes including Escherichia coli - the “workhorse” of the current biotechnology[195-198]. Prokaryotic glycosylation, however, differs significantly from that in eukaryotes. Therefore, it is preferable that naturally glycosylated human proteins are produced in eukaryotic cell lines. On the other hand, glycosylation in eukaryotes is species and cell specific[199]. Polysaccharides may vary in composition, chain length, binding sites on proteins and points of chain branching. This means that for the production of human proteins with preserved glycosylation pattern appropriate host cells have to be selected performing identical or similar glycosylation to that of the natural proteins[200] .
While native glycosylation of human therapeutic proteins is desirable, other non-enzymatic post-translational modifications should be avoided[201]. Spontaneous oxidation and deamination, sometimes followed by isomerization, are major causes of proteolysis during protein isolation, purification and storage. Deamination affects mainly asparagine residues and leads to their conversion into aspartate or iso-aspartate residues[202,203]. Glutamine in proteins may undergo similar spontaneous deamination to glutamate. Studies with oxidized and non-oxidized forms of IFNβ have shown that oxidized protein species are significantly more immunogenic[204]. The oxidation may lead to covalent aggregation of therapeutic proteins through the formation of cysteine and/or tyrosine covalent cross-links[54,205]. This is the reason why functionally unimportant Cys residues in therapeutic proteins are often replaced by alternative amino acids. To our knowledge, no one has tried so far to reduce covalent cross-linking of therapeutic proteins through replacement of Tyr residues. Last but not least, the leitmotif of the current review, the Maillard reaction, is another non-enzymatic post-translational modification of proteins, which contribution to the immunogenicity of protein drugs will be discussed in detail below.
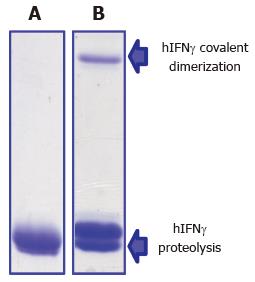
We entered the Maillard reaction field nearly 15 years ago when searching a reasonable explanation of the unexpected behavior of a cysteine-less variant of human interferon-gamma (hIFNγ) expressed in E. coli. Despite the lack of Cys residues, the protein underwent progressive covalent dimerization during storage under anaerobic conditions, which excluded oxidation (i.e., formation of disulfide bridges and tyrosine dimers) as a cause of the observed phenomenon (Figure 6). Brief literature survey led us to suppose that the Maillard reaction may provide a rational explanation of hIFNγ covalent dimerization and proteolysis. At that time, however, it was not yet clear whether the Maillard reaction may occur in prokaryotes because of their short life span and intense protein turnover. We undertook relevant investigations in this direction and found that both endogenous and recombinant proteins[73,206] as well as chromosomal DNA[207] of E. coli are involved in glycation under normal physiological conditions. The short life span of E. coli does not disagree with these observations. During batch fermentation of E coli a generation time of ca. 20 min and a few divisions are sufficient for the Maillard reaction to take off through the formation of Schiff bases in proteins. Then, the whole Maillard cascade of chemical transformations resulting in AGEs formation may happen in vitro during protein isolation, purification, formulation and storage. We indeed found that freshly isolated hIFNγ contains Amadri products but not AGEs, which accumulated in the protein over time accompanied by covalent aggregation, proteolysis and loss of biological activity[73,208]. Interferons are basic proteins rich in lysine and arginine residues and because of that susceptible to glycation. On the other hand, human interferons Type I (α and β) are the active ingredients of a number of pharmaceuticals used today for treatment of various cancers, autoimmune and viral diseases. For this reason we decided to analyze interferon-based pharmaceuticals for the presence of AGEs.
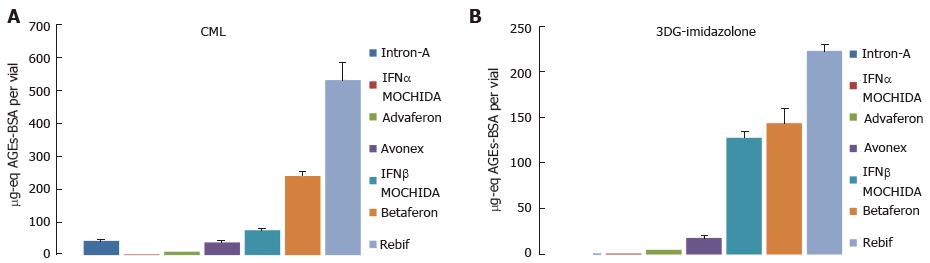
Type I interferon pharmaceuticals manufactured by different companies worldwide were tested: E. coli-derived hIFNα-2b (Intron-A, Schering-Plough Corp., Kenilworth, NJ, United States), natural (lymphoblast) nhIFNα (IFNα MOCHIDA1000, Mochida Pharmaceutical Co., Ltd, Tokyo, Japan), E. coli-derived hIFNα-con1 (Advaferon-1800, Yamanouchi Pharmaceutical Co., Ltd., Tokyo, Japan), natural (fibroblast) nhIFNβ (IFNβ MOCHIDA600, Mochida Pharamceutical Co., Ltd, Tokyo, Japan), E. coli-derived hIFNβ-1b (Betaferon, Schering AG, Berlin, Germany), CHO-derived IFNβ-1a (Rebif 44 mg, New Formulation, Merck Serono, Bari, Italy), and CHO-derived IFNβ-1a (Avonex 30 mg, Biogen Idec Inc., Weston, MA, United States). All pharmaceuticals except Adaferon and Rebif were powders containing HSA as a stabilizer while Advaferon and Rebif were liquid formulations without protein excipients. We tested all drugs for AGEs by enzyme-linked immunosorbent assay (ELISA) using two monoclonal antibodies specific for CML and 3DG-derived imidazolone (Figure 7). Lymphoblast IFNα MOCHIDA was negative for both CML and 3DG-imidazolone, whereas the content of both AGEs in fibroblast IFNβ MOCHIDA was higher than that in the E-coli-derived Intron-A and Advaferon. Also, Rebif produced in Chinese hamster ovary (CHO) cells proved to contain higher levels of AGEs compared to the E. coli-derived Betaferon. Based on these observations we concluded that therapeutic proteins are affected by glycation independently of whether they are produced by native or recombinant (either pro- or eukaryotic) cells. This result is not surprising bearing in mind both pro- and eukaryotic cells are glycation-proficient. Whether there are any differences in the glycation power between pro- and eukaryotic cells is a largely unexplored issue and a direction for future investigations. We suggest that the variable AGEs content in the drugs reflects different manufacturing technologies rather than specific characteristics of the cell-producers.
Five of the pharmaceuticals are formulated with HSA at concentrations ten to hundred times higher than that of interferons. Thus the reasonable question emerged whether AGEs, we are measuring, are located in HSA or in interferons. The answer of this question is “in interferons” because of the following reasons: (1) a highly purified pharmaceutical grade HSA is used for drugs’ formulation. Own mass-spectral analyses of all five HSA containing drugs confirmed the low glycation status of HSA[209], although 50% of HSA proved to be cysteinylated as shown also by other authors[210]. How HSA-Cys impacts drug stability is not yet clear and should be outlined as another direction for future investigations; (2) we have isolated an IFNβ enriched fraction from Betaferon by size-exclusion HPLC, which demonstrated 15 times higher concentration of CML as compared to Betaferon’s HSA[211]; and (3) as seen in Figure 7, the HSA-free drug Rebif demonstrated the highest concentration of CML and 3DG-imidazolone among all seven drugs while the HSA containing IFNα MOCHIDA was deprived of these two AGEs.
AGEs and immunogenicity of IFNβpharmaceuticals
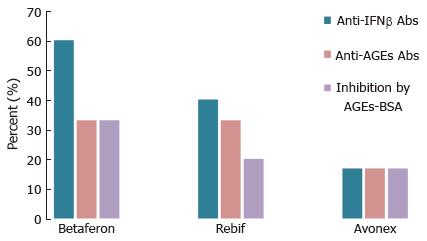
In most cases AGEs are bulky chemical moieties that may behave like haptens rendering self-proteins immunogenic. As seen in Figure 7, the four IFNβ pharmaceuticals tested proved to contain more CML and 3DG-imidazolone than the three IFNα drugs. Also, a number of studies report on immunogenicity of IFNβ[2,212-227]. Therefore, to either verify or reject the hypothesis that AGEs contribute to IFNβ immunogenicity, we studied sera from patients suffering from relapsing-remitting multiple sclerosis (MS) treated with Avonex (12 patients), Betaferon (15 patients) or Rebif (15 patients)[211]. Seventy four percent (31/42) of the patients enrolled in our study had flu-like symptoms, injection-site reactions, or both. Sera were tested for binding anti-IFNβ and anti-AGEs Abs of IgG isotype by direct ELISA. The percentage of anti-IFNβ Abs positive (Abs+) patients was highest in the Betaferon group (60%) followed by the Rebif (40%) and Avonex (17%) groups (Figure 8), which is consistent with data about the relative incidence of persistent neutralizing Abs (NAb) in patients treated with the same drugs[228]. Although CML and 3DG-imidazolone levels were higher in Rebif (Figure 7), Betaferon demonstrated higher immunogenicity than Rebif. Note that besides AGEs-modifications, and in contrast to the CHO-derived IFNβ1a (Rebif), the E. coli-derived IFNβ1b (Betaferon) lacks glycosylation at Asn-80, has an amino acid substitution (Ser17 for Cys17) and is prone to non-covalent aggregation[229], which could explain its higher immunogenicity. Seventeen percent (2/12) of the patients in the Avonex group were anti-AGEs Abs+, whereas for the other two groups (Betafeon and Rebif) the percentage of anti-AGEs Abs+ patients was 20% (3/15). All anti-AGEs Abs+ patients were anti-IFNβ Abs+. This, however, does not necessarily mean that the formation of anti-AGEs Abs in these patients was provoked by the IFNβ therapy. Convincing evidence for the link between the formation of anti-IFNβ and anti-AGEs Abs was obtained by competitive ELISA. The addition of an external AGEs-competitor (AGEs-BSA) to sera inhibited binding to IFNβ of two (Avonex), three (Rebif) and five (Betaferon) sera with inhibition ranging from 9% to 70%. The different degree of inhibition most likely reflects the relative contribution of AGEs to the overall IFNβ immunogenicity in each particular patient. All ten sera responding to inhibition by AGEs-BSA were tested also for response to inhibition by mAbs raised against CML and 3DG-imidazolone. Significant inhibition (P < 0.05) of sera reactivity to IFNβ was obtained with one serum from the Avonex group and with two sera from the Rebif group. One can suggest that in the remaining seven anti-AGEs Abs+ sera, which did not respond to inhibition by the two mAbs, the anti-AGEs Abs were raised against other AGEs (not CML and 3-DG-imidazolone) in IFNβ.
Whether the anti-AGEs Abs that cross-react with IFNβ neutralize drug activity and/or contribute to the observed inflammatory side reactions in treated patients are questions to be addressed in the future. Also, besides injection-site reactions and flu-like symptoms, cases of allergy[13-15] and anaphylaxis[11,16] have been reported for MS patients on IFNβ therapy. Therefore, it is worth to conduct case studies with such patients in order to answer the question of whether AGEs in IFNβ have contributed to the observed hypersensitivity reactions.
AGEs are formed in the human body under normal and pathological conditions, and are consumed with food as well. Because of the negative impact of AGEs on human physiology, elaborate mechanisms do exist for anti-AGEs defense ranging from enzymatic detoxification of AGEs’ precursors (i.e., carbonyl compounds[230-234], Schiff bases[235] and Amadori products[236]) going through intracellular proteasome/endosome degradation of protein AGEs, and ending up with renal clearance of free and peptide AGEs. Anti-AGEs antibodies have been detected in sera of healthy individuals as part of a homeostatic mechanism, which clears altered structures via in situ destruction or via opsonization[237]. The formation of AGEs starts during embryonic development and one can assume that against some stable AGEs in long-lived proteins an immune tolerance might be developed. IFNβ, however, is a short-lived protein (half-life of hours) and it is expected that upon administration in patients IFNβ-AGEs would be recognized as non-self-antigen thus provoking a classical type immune response.
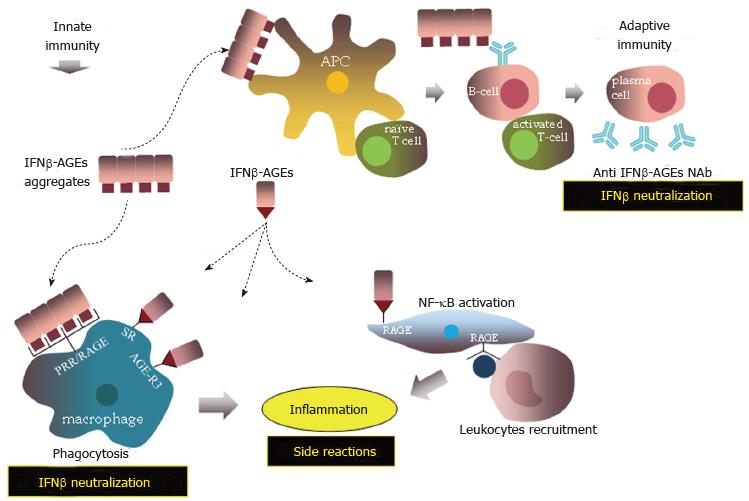
The adaptive immunity against IFNβ may be preceded by activation of the innate immunity. The binding of IFNβ-AGEs to non-specific (for AGEs) cellular receptors, such as the scavenger receptors and AGE-R3 on macrophages can result in IFNβ-AGEs degradation by phagocytosis (Figure 9). On the other hand, it has been shown that the specific receptor for AGEs, RAGE, belongs to the immunoglobulin superfamily and has the properties of a pattern recognition receptor (PRR)[137,238,239], which can sense recurring structures of the type of PAMPs[150]. It is well-known that the interaction of PRR with PAMPs triggers an intracellular signaling cascade, which initiates an innate, inflammatory by nature, immune response[240] resulting in degradation of the pathogen and in enhancement of the adaptive immune response. We have shown that Advaferon, Betaferon and Rebif contain covalent IFNβ aggregates[211,241], which are formed perhaps by glycoxidation via the mediator role of crosslinking AGEs like GOLD, MOLD, pentosidine etc. Such covalent aggregates may resemble PAMPs thus interacting with RAGE on macrophages, natural killer (NK) cells and other immune cells to elicit an innate immune response.
RAGE may play further role in inflammatory reactions[151,242] during IFNβ-AGEs therapy. It has been reported that CML[243,244] and MG-derived hydroimidazolone[245] are ligands for RAGE. Binding of RAGE with these and other AGEs and non-AGEs ligands such amfoterin does not result in ligands’ degradation but rather triggers different signaling pathways, including proinflammatory responses via the activation of transcription factor NF-κB[138,246,247]. Also, RAGE on endothelial cells may act as an adhesive receptor interacting with leukocyte β2 integrins[151] to recruit immune cells into sites of inflammation. Taken together the different pathways, in which the immune system may react against IFNβ-AGEs, are expected to result in neutralization of IFNβ activity (inefficient therapy), and in immune-mediated side effects (risky therapy).
Many proteins used today in medicine (erythropoietin, insulin, human growth hormone, clotting factors VIII and IX) have been shown to elicit varying incidence of antibody generation in treated patients[5]. Proteins are susceptible to glycation and it is the protein structure that determines to what extent given protein will accumulate AGEs. Scaling up the studies by including other therapeutic proteins will reveal the magnitude of AGEs as a causal factor of drugs’ immunogenicity. On the other side, not only the protein structure but the whole manufacturing process may impact the glycation status of proteins. Therefore, specific anti-glycation strategies have to be applied to prevent protein glycoixidation. The choice and engineering of appropriate host cells are of particular importance. The glycation potential of host cells should be controlled at all stages of the Maillard reaction including its initiation, propagation and progression. Engineering host cells to overexpress enzymes that detoxify carbonyl compounds will block glycation initiation and its propagation. In a similar way, the overexpression of deglycases[235,236] in host cells may interfere with early glycation stages. Also, a number of studies report on synthetic and natural compounds with anti-glycation activity[248]. Such substances can be added to the fermentation media of host cells to inhibit protein glycation. Fermentation parameters such as glucose concentration[249], oxygen supply (when applicable), temperature, and pH-control are also of particular relevance. If despite all these measures there are glycation adducts still left on proteins, additional techniques could be applied such as: (1) boronate affinity chromatography for extraction of protein species modified by early glycation products[250]; and (2) use of AGEs-breakers[251,252] for removal of AGEs. Although costly, isolation, purification, formulation and storage of proteins are best to be conducted under anaerobic conditions, thereby avoiding the use of carbonyl compounds and transition metal ions at each step. Finally, health care authorities including the American Food and Drug Administration and the European Medical Agency are recommended to undertake relevant initiatives for AGEs assessment of protein drugs.
P- Reviewer: Ferrante A, Kwon HJ, Nagata T S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Foged C, Sundblad A. Immune reactions towards biopharmaceuticals – a general mechanistic overview. Immunogenicity of Biopharmaceuticals. New York: Springer 2008; 1-25. [DOI] [Full Text] |
| 2. | Bertolotto A, Deisenhammer F, Gallo P, Sölberg Sørensen P. Immunogenicity of interferon beta: differences among products. J Neurol. 2004;251 Suppl 2:II15-II24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Albertsson-Wikland K. Clinical trial with authentic recombinant somatropin in Sweden and Finland. Acta Paediatr Scand Suppl. 1987;331:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Takano K, Shizume K, Hibi I. Turner’s syndrome: treatment of 203 patients with recombinant human growth hormone for one year. A multicentre study. Acta Endocrinol (Copenh). 1989;120:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Schellekens H, Casadevall N. Immunogenicity of recombinant human proteins: causes and consequences. J Neurol. 2004;251 Suppl 2:II4-II9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 778] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 7. | Locatelli F, Aljama P, Barany P, Canaud B, Carrera F, Eckardt KU, Macdougall IC, Macleod A, Hörl WH, Wiecek A. Erythropoiesis-stimulating agents and antibody-mediated pure red-cell aplasia: here are we now and where do we go from here. Nephrol Dial Transplant. 2004;19:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Kuter DJ. Future directions with platelet growth factors. Semin Hematol. 2000;37:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Weber RW. Adverse reactions to biological modifiers. Curr Opin Allergy Clin Immunol. 2004;4:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Frost H. Antibody-mediated side effects of recombinant proteins. Toxicology. 2005;209:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Corona T, Leon C, Ostrosky-Zeichner L. Severe anaphylaxis with recombinant interferon beta. Neurology. 1999;52:425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lusher JM. Inhibitor antibodies to factor VIII and factor IX: management. Semin Thromb Hemost. 2000;26:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Fusun Kalpaklioglu A, Baccioglu Kavut A, Erdemoglu AK. Desensitization in interferon-beta1a allergy: a case report. Int Arch Allergy Immunol. 2009;149:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Rosa DJ, Matias Fde A, Cedrim SD, Machado RF, Sá AA, Silva VC. Acute acneiform eruption induced by interferon beta-1b during treatment for multiple sclerosis. An Bras Dermatol. 2011;86:336-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Guijarro C, Benito-León J, Bermejo-Pareja F. Widespread urticaria due to intramuscular interferon beta-1a therapy for multiple sclerosis. Neurol Sci. 2011;32:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Cortellini G, Amadori A, Comandini T, Corvetta A. Interferon beta 1a anaphylaxis, a case report. Standardization of non-irritating concentration for allergy skin tests. Eur Ann Allergy Clin Immunol. 2013;45:181-182. [PubMed] |
| 17. | Smith LF. Species variation in the amino acid sequence of insulin. Am J Med. 1966;40:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 276] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Berson SA, Yalow RS, Bauman A, Rothschild MA, Newerly K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J Clin Invest. 1956;35:170-190. [PubMed] |
| 19. | Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med. 2005;72:250-256. [PubMed] |
| 20. | Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 21. | Du X, Tang JG. Hydroxyl group of insulin A19Tyr is essential for receptor binding: studies on (A19Phe)insulin. Biochem Mol Biol Int. 1998;45:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W. Structure-immunogenicity relationships of therapeutic proteins. Pharm Res. 2004;21:897-903. [PubMed] |
| 23. | Braun A, Kwee L, Labow MA, Alsenz J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-alpha) in normal and transgenic mice. Pharm Res. 1997;14:1472-1478. [PubMed] |
| 24. | Villalobos AP, Gunturi SR, Heavner GA. Interaction of polysorbate 80 with erythropoietin: a case study in protein-surfactant interactions. Pharm Res. 2005;22:1186-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Haselbeck A. Epoetins: differences and their relevance to immunogenicity. Curr Med Res Opin. 2003;19:430-432. [PubMed] |
| 26. | Maillard LC. Action des acides amines sur les sucres: formation des melanoidines par voie methodique. C R Acad Sci. 1912;154:66-68. |
| 27. | Rahbar S. An abnormal hemoglobin in red cells of diabetics. Clin Chim Acta. 1968;22:296-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 233] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Rahbar S. The discovery of glycated hemoglobin: a major event in the study of nonenzymatic chemistry in biological systems. Ann N Y Acad Sci. 2005;1043:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Bookchin RM, Gallop PM. Structure of hemoglobin AIc: nature of the N-terminal beta chain blocking group. Biochem Biophys Res Commun. 1968;32:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 248] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Monnier VM, Stevens VJ, Cerami A. Maillard reactions involving proteins and carbohydrates in vivo: relevance to diabetes mellitus and aging. Prog Food Nutr Sci. 1981;5:315-327. [PubMed] |
| 31. | Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981;211:491-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 562] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 923] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 33. | Thorpe SR, Baynes JW. Role of the Maillard reaction in diabetes mellitus and diseases of aging. Drugs Aging. 1996;9:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 204] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Baynes JW. From life to death--the struggle between chemistry and biology during aging: the Maillard reaction as an amplifier of genomic damage. Biogerontology. 2000;1:235-246. [PubMed] |
| 35. | Baynes JW. The Maillard hypothesis on aging: time to focus on DNA. Ann N Y Acad Sci. 2002;959:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuvenation Res. 2006;9:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Nass N, Bartling B, Navarrete Santos A, Scheubel RJ, Börgermann J, Silber RE, Simm A. Advanced glycation end products, diabetes and ageing. Z Gerontol Geriatr. 2007;40:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications. Diabetes Obes Metab. 2007;9:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 39. | Kuhn R, Weygand F. The Amadori rearrangement. Ber. 1937;70B:769-772. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Hodge JE. Deydrated Foods: Chemistry of Browning reactions in model systems. J Agric Food Chem. 1953;1:928-943. [DOI] [Full Text] |
| 41. | Cerami A. Aging of proteins and nucleic acids: what is the role of glucose. TIBS. 1986;11:311-314. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Anet EFLJ. Degradation of carbohydrates. I. Isolation of 3-deoxyhexosones. Australian J Chem. 1960;13:396-403. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Kato H. Studies on browning reactions between sugars and amino compounds. V. Isolation and characterization of new carbonyl compounds, 3-deoxyglucosones formed from N-glycosides and their significance for browning reaction. Bull Agric Chem Soc Japan. 1960;24:1-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Hayashi T, Namiki M. Formation of two-carbon sugar fragments at an early stage of the browning reaction of sugar and amine. Agric Biol Chem. 1980;44:2575-2580. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Thornalley P, Wolff S, Crabbe J, Stern A. The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalysed by buffer ions. Biochim Biophys Acta. 1984;797:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J. 1987;245:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 882] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 47. | Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344 Pt 1:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 48. | Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982-9986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 557] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 49. | Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 2000;28:1708-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 373] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 50. | Nakayama T, Hayase F, Kato H. Formation of Nε-(2-formyl-5-hydroxy-methyl-pyrrol-1-yl)-L-norleucine in the Maillard reaction between D-glucose and L-lysine. Agric Biol Chem. 1980;44:1201-1202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Pongor S, Ulrich PC, Bencsath FA, Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci USA. 1984;81:2684-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 280] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Ahmed MU, Thorpe SR, Baynes JW. Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J Biol Chem. 1986;261:4889-4894. [PubMed] |
| 53. | Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem. 1989;264:21597-21602. [PubMed] |
| 54. | Ahmed N, Thornalley PJ. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem Soc Trans. 2003;31:1417-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Thornalley PJ, Battah S, Ahmed N, Karachalias N, Agalou S, Babaei-Jadidi R, Dawnay A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem J. 2003;375:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 533] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 56. | Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 394] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 57. | Lapolla A, Molin L, Traldi P. Protein Glycation in diabetes as determined by mass spectrometry. Int J Endocrinol. 2013;412103. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Han L, Li L, Li B, Zhao D, Li Y, Xu Z, Liu G. Review of the characteristics of food-derived and endogenous ne-carboxymethyllysine. J Food Prot. 2013;76:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Ledl F, Schleicher E. The Maillard reaction in food and human body. Angewandte Chemie. 1990;102:597-626. [RCA] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 306] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 61. | Hasenkopf K, Rönner B, Hiller H, Pischetsrieder M. Analysis of glycated and ascorbylated proteins by gas chromatography-mass spectrometry. J Agric Food Chem. 2002;50:5697-5703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 62. | Reddy S, Bichler J, Wells-Knecht KJ, Thorpe SR, Baynes JW. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872-10878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 366] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 63. | Miki Hayashi C, Nagai R, Miyazaki K, Hayase F, Araki T, Ono T, Horiuchi S. Conversion of Amadori products of the Maillard reaction to N(epsilon)-(carboxymethyl)lysine by short-term heating: possible detection of artifacts by immunohistochemistry. Lab Invest. 2002;82:795-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Konishi F, Hayase F, Kato H. Novel imidazolone compound formed by the advanced Maillard reaction of 3-deoxyglucosone and arginine residues in proteins. Biosci Biotech Biochem. 1994;58:1953-1955. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 276] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 66. | Ahmed N, Thornalley PJ. Chromatographic assay of glycation adducts in human serum albumin glycated in vitro by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and intrinsic fluorescence. Biochem J. 2002;364:15-24. [PubMed] |
| 67. | Argirova M. Biological effects of the Maillard reaction. DSc Thesis. 2015;Faculty of Pharmacy, Medical University of Plovdiv:8-9. |
| 68. | Ookawara T, Kawamura N, Kitagawa Y, Taniguchi N. Site-specific and random fragmentation of Cu,Zn-superoxide dismutase by glycation reaction. Implication of reactive oxygen species. J Biol Chem. 1992;267:18505-18510. [PubMed] |
| 69. | Islam KN, Takahashi M, Higashiyama S, Myint T, Uozumi N. Fragmentation of ceruloplasmin following non-enzymatic glycation reaction. J Biochem. 1995;118:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Li S, Patapoff TW, Overcashier D, Hsu C, Nguyen TH, Borchardt RT. Effects of reducing sugars on the chemical stability of human relaxin in the lyophilized state. J Pharm Sci. 1996;85:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Njoroge FG, Fernandes AA, Monnier VM. Mechanism of formation of the putative advanced glycosylation end product and protein cross-link 2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole. J Biol Chem. 1988;263:10646-10652. [PubMed] |
| 72. | Miyata T, Taneda S, Kawai R, Ueda Y, Horiuchi S, Hara M, Maeda K, Monnier VM. Identification of pentosidine as a native structure for advanced glycation end products in beta-2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc Natl Acad Sci USA. 1996;93:2353-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Mironova R, Niwa T, Dimitrova R, Boyanova M, Ivanov I. Glycation and post-translational processing of human interferon-gamma expressed in Escherichia coli. J Biol Chem. 2003;278:51068-51074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Wells-Knecht KJ, Brinkmann E, Baynes JW. Characterization of an imidazolium salt formed from glyoxal and N-Hypuryllysine: a model for Maillard reaction crosslinks in proteins. J Org Chem. 1995;60:6246-6247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Brinkmann E, Wells-Knecht KJ, Thorpe SR, Baynes JW. Characterization of an imidazolium compound formed by reaction of methylglyoxal and N-hyppuryllysine. J Chem Soc Perkin Trans. 1995;1:2817-2818. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Skovsted IC, Christensen M, Breinholt J, Mortensen SB. Characterisation of a novel AGE-compound derived from lysine and 3-deoxyglucosone. Cell Mol Biol (Noisy-le-grand). 1998;44:1159-1163. [PubMed] |
| 77. | Glomb MA, Pfahler C. Amides are novel protein modifications formed by physiological sugars. J Biol Chem. 2001;276:41638-41647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Sell DR, Monnier VM. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19:77-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Nemet I, Strauch CM, Monnier VM. Favored and disfavored pathways of protein crosslinking by glucose: glucose lysine dimer (GLUCOLD) and crossline versus glucosepane. Amino Acids. 2011;40:167-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Obayashi H, Nakano K, Shigeta H, Yamaguchi M, Yoshimori K, Fukui M, Fujii M, Kitagawa Y, Nakamura N, Nakamura K. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun. 1996;226:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Yamaguchi M, Nakamura N, Nakano K, Kitagawa Y, Shigeta H, Hasegawa G, Ienaga K, Nakamura K, Nakazawa Y, Fukui I. Immunochemical quantification of crossline as a fluorescent advanced glycation endproduct in erythrocyte membrane proteins from diabetic patients with or without retinopathy. Diabet Med. 1998;15:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Nakamura K, Nakazawa Y, Ienaga K. Acid-stable fluorescent advanced glycation end products: vesperlysines A, B, and C are formed as crosslinked products in the Maillard reaction between lysine or proteins with glucose. Biochem Biophys Res Commun. 1997;232:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Lederer MO, Klaiber RG. Cross-linking of proteins by Maillard processes: characterization and detection of lysine-arginine cross-links derived from glyoxal and methylglyoxal. Bioorg Med Chem. 1999;7:2499-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Biemel KM, Reihl O, Conrad J, Lederer MO. Formation pathways for lysine-arginine cross-links derived from hexoses and pentoses by Maillard processes: unraveling the structure of a pentosidine precursor. J Biol Chem. 2001;276:23405-23412. [PubMed] |
| 85. | Tressel R, Wondrak G, Kersten E, Rewicki D. Structure and potential crosslinking reactivity of a new pentose-specific Maillard product. J Agric Food Chem. 1994;42:2692-2697. [DOI] [Full Text] |
| 86. | Thornalley PJ. Physiological formation and processing of glycation adducts. Available from: http://www2.warwick.ac.uk/fac/med/research/tsm/mvhealth/proteindamage/physiology/formation/. |
| 87. | Yu Y, Thorpe SR, Jenkins AJ, Shaw JN, Sochaski MA, McGee D, Aston CE, Orchard TJ, Silvers N, Peng YG. Advanced glycation end-products and methionine sulphoxide in skin collagen of patients with type 1 diabetes. Diabetologia. 2006;49:2488-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, DeBolt E, Koke M, Hage DS. Review: Glycation of human serum albumin. Clin Chim Acta. 2013;425:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 89. | Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984;259:3812-3817. [PubMed] |
| 90. | Iberg N, Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986;261:13542-13545. [PubMed] |
| 91. | Shapiro R, McManus MJ, Zalut C, Bunn HF. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem. 1980;255:3120-3127. [PubMed] |
| 92. | Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 93. | Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027-39031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 647] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 94. | Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, Bayliss MT, Bijlsma JW, Lafeber FP, Tekoppele JM. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350 Pt 2:381-387. [PubMed] |
| 95. | Wells-Knecht MC, Lyons TJ, McCance DR, Thorpe SR, Baynes JW. Age-dependent increase in ortho-tyrosine and methionine sulfoxide in human skin collagen is not accelerated in diabetes. Evidence against a generalized increase in oxidative stress in diabetes. J Clin Invest. 1997;100:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Sanguineti R, Puddu A, Mach F, Montecucco F, Viviani GL. Advanced glycation end products play adverse proinflammatory activities in osteoporosis. Mediators Inflamm. 2014;2014:975872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 97. | Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324:565-570. [PubMed] |
| 98. | Greifenhagen U, Nguyen VD, Moschner J, Giannis A, Frolov A, Hoffmann R. Sensitive and site-specific identification of carboxymethylated and carboxyethylated peptides in tryptic digests of proteins and human plasma. J Proteome Res. 2015;14:768-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci. 2003;44:5287-5292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Michalek MT, Grant EP, Rock KL. Chemical denaturation and modification of ovalbumin alters its dependence on ubiquitin conjugation for class I antigen presentation. J Immunol. 1996;157:617-624. [PubMed] |
| 101. | Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1579] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 102. | DeGroot J, Verzijl N, Wenting-Van Wijk MJ, Bank RA, Lafeber FP, Bijlsma JW, TeKoppele JM. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 2001;44:2562-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 103. | Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990;173:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 446] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 104. | Tsai CS, White JH. Activation of liver alcohol dehydrogenase by glycosylation. Biochem J. 1983;209:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Niwa T, Tsukushi S. 3-deoxyglucosone and AGEs in uremic complications: inactivation of glutathione peroxidase by 3-deoxyglucosone. Kidney Int Suppl. 2001;78:S37-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Monti P, Brigatti C, Krasmann M, Ziegler AG, Bonifacio E. Concentration and activity of the soluble form of the interleukin-7 receptor α in type 1 diabetes identifies an interplay between hyperglycemia and immune function. Diabetes. 2013;62:2500-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 107. | Giardino I, Edelstein D, Brownlee M. Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity. A model for intracellular glycosylation in diabetes. J Clin Invest. 1994;94:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 108. | Acosta J, Hettinga J, Flückiger R, Krumrei N, Goldfine A, Angarita L, Halperin J. Molecular basis for a link between complement and the vascular complications of diabetes. Proc Natl Acad Sci USA. 2000;97:5450-5455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 109. | Chellan P, Nagaraj RH. Early glycation products produce pentosidine cross-links on native proteins. novel mechanism of pentosidine formation and propagation of glycation. J Biol Chem. 2001;276:3895-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 654] [Cited by in RCA: 854] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 111. | Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab. 2014;3:94-108. [PubMed] |
| 112. | Bulteau AL, Verbeke P, Petropoulos I, Chaffotte AF, Friguet B. Proteasome inhibition in glyoxal-treated fibroblasts and resistance of glycated glucose-6-phosphate dehydrogenase to 20 S proteasome degradation in vitro. J Biol Chem. 2001;276:45662-45668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 113. | Höhn A, Jung T, Grune T. Pathophysiological importance of aggregated damaged proteins. Free Radic Biol Med. 2014;71:70-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 114. | Grimm S, Ott C, Hörlacher M, Weber D, Höhn A, Grune T. Advanced-glycation-end-product-induced formation of immunoproteasomes: involvement of RAGE and Jak2/STAT1. Biochem J. 2012;448:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 115. | Henle T. AGEs in foods: do they play a role in uremia. Kidney Int Suppl. 2003;S145-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 116. | Lee K, Erbersdobler HF. In: Labuza TP, Reineccius GA, Monnier VM, O’Brien J and Baynes JW, eds. Maillard Reaction in Chemistry, Food and Health. UK: The Royal Society of Chemistry Cambridge 1994; 358-363. |
| 117. | Finot PA. Metabolism and physiological effects of Maillard reactions products. The Maillard reaction in food processing, human nutrition and physiology. Basel: Birkhäuser 1990; 259-272. |
| 118. | Wiame E, Delpierre G, Collard F, Van Schaftingen E. Identification of a pathway for the utilization of the Amadori product fructoselysine in Escherichia coli. J Biol Chem. 2002;277:42523-42529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 119. | Finot PA, Magnenat E, Mottu F, Bujard E. Biologic availability and metabolic transit of amino acids modified by technological processing. Ann Nutr Aliment. 1978;32:326-338. [PubMed] |
| 120. | Oste RE, Miller R, Sjorstrom H, Noren O. Effect os Maillard reaction-products on protein digestion-studies on pure compounds. J Agric Food Chem. 1987;35:938-942. [DOI] [Full Text] |
| 121. | Ahmed N, Thornalley PJ, Lüthen R, Häussinger D, Sebekova K, Schinzel R, Voelker W, Heidland A. Processing of protein glycation, oxidation and nitrosation adducts in the liver and the effect of cirrhosis. J Hepatol. 2004;41:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Liardon R, De Weck-Gaudard D, Philippossian G, Finot P-A. Identification of N-carboxymethyllysine: a new Maillard reaction product, in rat urine. J Agr Food Chem. 1987;35:427-431. [DOI] [Full Text] |
| 123. | Germond JE, Philippossian G, Richli U, Bracco I, Arnaud MJ. Rapid and complete urinary elimination of [14C]-5-hydroxymethyl-2-furaldehyde administered orally or intravenously to rats. J Toxicol Environ Health. 1987;22:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 1997;94:6474-6479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 578] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 125. | Yang Z, Makita Z, Horii Y, Brunelle S, Cerami A, Sehajpal P, Suthanthiran M, Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991;174:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 126. | Li YM, Mitsuhashi T, Wojciechowicz D, Shimizu N, Li J, Stitt A, He C, Banerjee D, Vlassara H. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci USA. 1996;93:11047-11052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 246] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 127. | Vlassara H, Li YM, Imani F, Wojciechowicz D, Yang Z, Liu FT, Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995;1:634-646. [PubMed] |
| 128. | Prabhudas M, Bowdish D, Drickamer K, Febbraio M, Herz J, Kobzik L, Krieger M, Loike J, Means TK, Moestrup SK. Standardizing scavenger receptor nomenclature. J Immunol. 2014;192:1997-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 129. | Zani IA, Stephen SL, Mughal NA, Russell D, Homer-Vanniasinkam S, Wheatcroft SB, Ponnambalam S. Scavenger receptor structure and function in health and disease. Cells. 2015;4:178-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 130. | el Khoury J, Thomas CA, Loike JD, Hickman SE, Cao L, Silverstein SC. Macrophages adhere to glucose-modified basement membrane collagen IV via their scavenger receptors. J Biol Chem. 1994;269:10197-10200. [PubMed] |
| 131. | Araki N, Higashi T, Mori T, Shibayama R, Kawabe Y, Kodama T, Takahashi K, Shichiri M, Horiuchi S. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur J Biochem. 1995;230:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 132. | Ohgami N, Nagai R, Miyazaki A, Ikemoto M, Arai H, Horiuchi S, Nakayama H. Scavenger receptor class B type I-mediated reverse cholesterol transport is inhibited by advanced glycation end products. J Biol Chem. 2001;276:13348-13355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 133. | Ohgami N, Nagai R, Ikemoto M, Arai H, Miyazaki A, Hakamata H, Horiuchi S, Nakayama H. CD36, serves as a receptor for advanced glycation endproducts (AGE). J Diabetes Complications. 2002;16:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 134. | Jono T, Miyazaki A, Nagai R, Sawamura T, Kitamura T, Horiuchi S. Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) serves as an endothelial receptor for advanced glycation end products (AGE). FEBS Lett. 2002;511:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 135. | Tamura Y, Adachi H, Osuga J, Ohashi K, Yahagi N, Sekiya M, Okazaki H, Tomita S, Iizuka Y, Shimano H. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J Biol Chem. 2003;278:12613-12617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 136. | Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998-15004. [PubMed] |
| 137. | Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 851] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 138. | Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 139. | Gefter JV, Shaufl AL, Fink MP, Delude RL. Comparison of distinct protein isoforms of the receptor for advanced glycation end-products expressed in murine tissues and cell lines. Cell Tissue Res. 2009;337:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 140. | Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 1762] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 141. | Sturchler E, Galichet A, Weibel M, Leclerc E, Heizmann CW. Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J Neurosci. 2008;28:5149-5158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 142. | Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752-25761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 914] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 143. | Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 967] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 144. | Müller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, Beltrame M, Bianchi ME. New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337-4340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 351] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 145. | Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111-130. [PubMed] |
| 146. | Akirav EM, Preston-Hurlburt P, Garyu J, Henegariu O, Clynes R, Schmidt AM, Herold KC. RAGE expression in human T cells: a link between environmental factors and adaptive immune responses. PLoS One. 2012;7:e34698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 147. | Akirav EM, Henegariu O, Preston-Hurlburt P, Schmidt AM, Clynes R, Herold KC. The receptor for advanced glycation end products (RAGE) affects T cell differentiation in OVA induced asthma. PLoS One. 2014;9:e95678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 148. | Ilchmann A, Burgdorf S, Scheurer S, Waibler Z, Nagai R, Wellner A, Yamamoto Y, Yamamoto H, Henle T, Kurts C. Glycation of a food allergen by the Maillard reaction enhances its T-cell immunogenicity: role of macrophage scavenger receptor class A type I and II. J Allergy Clin Immunol. 2010;125:175-83.e1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 149. | Heilmann M, Wellner A, Gadermaier G, Ilchmann A, Briza P, Krause M, Nagai R, Burgdorf S, Scheurer S, Vieths S. Ovalbumin modified with pyrraline, a Maillard reaction product, shows enhanced T-cell immunogenicity. J Biol Chem. 2014;289:7919-7928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 150. | Janeway CA. Approaching the asymptote Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2100] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 151. | Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004;6:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 152. | Reiniger N, Lau K, McCalla D, Eby B, Cheng B, Lu Y, Qu W, Quadri N, Ananthakrishnan R, Furmansky M. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 153. | Ramasamy R, Yan SF, Schmidt AM. Advanced glycation endproducts: from precursors to RAGE: round and round we go. Amino Acids. 2012;42:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 154. | Baydanoff S, Nicoloff G, Alexiev C. Age-dependent changes in the level of antielastin antibodies of different immunoglobulin classes (IgG, IgM, IgA and IgD) in the human serum. Cor Vasa. 1991;33:197-205. [PubMed] |
| 155. | Mir AR, Moinuddin S, Khan F, Alam K, Ali A. Structural changes in histone H2A by methylglyoxal generate highly immunogenic amorphous aggregates with implications in auto-immune response in cancer. Glycobiology. 2016;26:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 156. | Arif B, Ashraf JM, Moinuddin J, Arif Z, Alam K. Structural and immunological characterization of Amadori-rich human serum albumin: role in diabetes mellitus. Arch Biochem Biophys. 2012;522:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 157. | Ansari NA, Moinuddin K, Ali A. Preferential recognition of Amadori-rich lysine residues by serum antibodies in diabetes mellitus: role of protein glycation in the disease process. Hum Immunol. 2009;70:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 158. | Rasheed Z, Kumar L, Sajid , Prasad I, Ansari NA, Ahmad R. Advanced Glycation End Products (AGEs) damaged IgG, a target for circulating autoantibodies in patients with type 1 diabetes mellitus. Open Glycosci. 2009;2:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 159. | Shahab U, Tabrez S, Khan MS, Akhter F, Khan MS, Saeed M, Ahmad K, Srivastava AK, Ahmad S. Immunogenicity of DNA-advanced glycation end product fashioned through glyoxal and arginine in the presence of Fe³⁺: its potential role in prompt recognition of diabetes mellitus auto-antibodies. Chem Biol Interact. 2014;219:229-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 160. | Ahmad S, Moinuddin K, Shahab U, Alam K, Ali A. Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+. Biochem Biophys Res Commun. 2011;407:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 161. | Akhter F, Khan MS, Singh S, Ahmad S. An immunohistochemical analysis to validate the rationale behind the enhanced immunogenicity of D-ribosylated low density lipo-protein. PLoS One. 2014;9:e113144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 162. | Akhter F, Khan MS, Ahmad S. Acquired immunogenicity of calf thymus DNA and LDL modified by D-ribose: a comparative study. Int J Biol Macromol. 2015;72:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 163. | Khan MW, Qadrie ZL, Khan WA. Antibodies against gluco-oxidatively modified human serum albumin detected in diabetes-associated complications. Int Arch Allergy Immunol. 2010;153:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 164. | Shibayama R, Araki N, Nagai R, Horiuchi S. Autoantibody against N(epsilon)-(carboxymethyl)lysine: an advanced glycation end product of the Maillard reaction. Diabetes. 1999;48:1842-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 165. | Bassiouny AR, Rosenberg H, McDonald TL. Glucosylated collagen is antigenic. Diabetes. 1983;32:1182-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 166. | Vay D, Vidali M, Allochis G, Cusaro C, Rolla R, Mottaran E, Bellomo G, Albano E. Antibodies against advanced glycation end product Nepsilon-(carboxymethyl)lysine in healthy controls and diabetic patients. Diabetologia. 2000;43:1385-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 167. | Nicoloff G, Baydanoff S, Petrova Ch, Christova P. Antibodies to advanced glycation end products in children with diabetes mellitus. Vascul Pharmacol. 2002;39:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 168. | Buongiorno AM, Morelli S, Sagratella E, Cipriani R, Mazzaferro S, Morano S, Sensi M. Immunogenicity of advanced glycation end products in diabetic patients and in nephropathic non-diabetic patients on hemodialysis or after renal transplantation. J Endocrinol Invest. 2008;31:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 169. | Turk Z, Ljubic S, Turk N, Benko B. Detection of autoantibodies against advanced glycation endproducts and AGE-immune complexes in serum of patients with diabetes mellitus. Clin Chim Acta. 2001;303:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 170. | Ligier S, Fortin PR, Newkirk MM. A new antibody in rheumatoid arthritis targeting glycated IgG: IgM anti-IgG-AGE. Br J Rheumatol. 1998;37:1307-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Tai AW, Newkirk MM. An autoantibody targeting glycated IgG is associated with elevated serum immune complexes in rheumatoid arthritis (RA). Clin Exp Immunol. 2000;120:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 172. | Foged C, Sundblad A. Case Study: Immunogenicity of Anti-TNF Antibodies. Immunogenicity of Biopharmaceuticals. New York: Springer 2008; 189-203. [DOI] [Full Text] |
| 173. | Zhu L, He Z, Wu F, Ding R, Jiang Q, Zhang J, Fan M, Wang X, Eva B, Jan N. Immunization with advanced glycation end products modified low density lipoprotein inhibits atherosclerosis progression in diabetic apoE and LDLR null mice. Cardiovasc Diabetol. 2014;13:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 174. | Lopes-Virella MF, Virella G. Clinical significance of the humoral immune response to modified LDL. Clin Immunol. 2010;134:55-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 175. | Virella G, Thorpe SR, Alderson NL, Derrick MB, Chassereau C, Rhett JM, Lopes-Virella MF. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J Lipid Res. 2004;45:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 176. | Hunt KJ, Baker N, Cleary P, Backlund JY, Lyons T, Jenkins A, Virella G, Lopes-Virella MF. Oxidized LDL and AGE-LDL in circulating immune complexes strongly predict progression of carotid artery IMT in type 1 diabetes. Atherosclerosis. 2013;231:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 177. | Baynes BM, Wang DI, Trout BL. Role of arginine in the stabilization of proteins against aggregation. Biochemistry. 2005;44:4919-4925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 178. | Tobler SA, Fernandez EJ. Structural features of interferon-gamma aggregation revealed by hydrogen exchange. Protein Sci. 2002;11:1340-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 179. | Uversky VN, Segel DJ, Doniach S, Fink AL. Association-induced folding of globular proteins. Proc Natl Acad Sci USA. 1998;95:5480-5483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 180. | Moore WV, Leppert P. Role of aggregated human growth hormone (hGH) in development of antibodies to hGH. J Clin Endocrinol Metab. 1980;51:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 181. | Palleroni AV, Aglione A, Labow M, Brunda MJ, Pestka S, Sinigaglia F, Garotta G, Alsenz J, Braun A. Interferon immunogenicity: preclinical evaluation of interferon-alpha 2a. J Interferon Cytokine Res. 1997;17 Suppl 1:S23-S27. [PubMed] |
| 182. | Hochuli E. Interferon immunogenicity: technical evaluation of interferon-alpha 2a. J Interferon Cytokine Res. 1997;17 Suppl 1:S15-S21. [PubMed] |
| 183. | Ryff JC. Clinical investigation of the immunogenicity of interferon-alpha 2a. J Interferon Cytokine Res. 1997;17 Suppl 1:S29-S33. [PubMed] |
| 184. | Hermeling S, Aranha L, Damen JM, Slijper M, Schellekens H, Crommelin DJ, Jiskoot W. Structural characterization and immunogenicity in wild-type and immune tolerant mice of degraded recombinant human interferon alpha2b. Pharm Res. 2005;22:1997-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 185. | Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;11:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 423] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 186. | Hermeling S, Schellekens H, Maas C, Gebbink MF, Crommelin DJ, Jiskoot W. Antibody response to aggregated human interferon alpha2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J Pharm Sci. 2006;95:1084-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 187. | Purohit VS, Balasubramanian SV. Interaction of dicaproyl phosphatidylserine with recombinant factor VIII and its impact on immunogenicity. AAPS J. 2006;8:E362-E370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 188. | Bachmann MF, Oxenius A, Speiser DE, Mariathasan S, Hengartner H, Zinkernagel RM, Ohashi PS. Peptide-induced T cell receptor down-regulation on naive T cells predicts agonist/partial agonist properties and strictly correlates with T cell activation. Eur J Immunol. 1997;27:2195-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 189. | Bhanot G. Results from modeling of B-Cell receptors binding to antigen. Prog Biophys Mol Biol. 2004;85:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 190. | Dintzis RZ, Okajima M, Middleton MH, Greene G, Dintzis HM. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989;143:1239-1244. [PubMed] |
| 191. | Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:E501-E507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1011] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 192. | Smith RA, Young J, Weis JJ, Weis JH. Expression of the mouse fragilis gene products in immune cells and association with receptor signaling complexes. Genes Immun. 2006;7:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 193. | Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MF. A role for protein misfolding in immunogenicity of biopharmaceuticals. J Biol Chem. 2007;282:2229-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 194. | Sulzer B, Perelson AS. Immunons revisited: binding of multivalent antigens to B cells. Mol Immunol. 1997;34:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 195. | Lindenthal C, Elsinghorst EA. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect Immun. 1999;67:4084-4091. [PubMed] |
| 196. | Sherlock O, Dobrindt U, Jensen JB, Munk Vejborg R, Klemm P. Glycosylation of the self-recognizing Escherichia coli Ag43 autotransporter protein. J Bacteriol. 2006;188:1798-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 197. | Gross J, Grass S, Davis AE, Gilmore-Erdmann P, Townsend RR, St Geme JW. The Haemophilus influenzae HMW1 adhesin is a glycoprotein with an unusual N-linked carbohydrate modification. J Biol Chem. 2008;283:26010-26015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 198. | Cuccui J, Wren B. Hijacking bacterial glycosylation for the production of glycoconjugates, from vaccines to humanised glycoproteins. J Pharm Pharmacol. 2015;67:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 199. | Kagawa Y, Takasaki S, Utsumi J, Hosoi K, Shimizu H, Kochibe N, Kobata A. Comparative study of the asparagine-linked sugar chains of natural human interferon-beta 1 and recombinant human interferon-beta 1 produced by three different mammalian cells. J Biol Chem. 1988;263:17508-17515. [PubMed] |
| 200. | Goochee CF, Monica T. Environmental effects on protein glycosylation. Biotechnology (N Y). 1990;8:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 201. | Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10:307-377. [PubMed] |
| 202. | Xie M, Schowen RL. Secondary structure and protein deamidation. J Pharm Sci. 1999;88:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 203. | Lai MC, Topp EM. Solid-state chemical stability of proteins and peptides. J Pharm Sci. 1999;88:489-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 204. | van Beers MM, Sauerborn M, Gilli F, Brinks V, Schellekens H, Jiskoot W. Oxidized and aggregated recombinant human interferon beta is immunogenic in human interferon beta transgenic mice. Pharm Res. 2011;28:2393-2402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 205. | Giulivi C, Davies KJ. Mechanism of the formation and proteolytic release of H2O2-induced dityrosine and tyrosine oxidation products in hemoglobin and red blood cells. J Biol Chem. 2001;276:24129-24136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 206. | Mironova R, Niwa T, Hayashi H, Dimitrova R, Ivanov I. Evidence for non-enzymatic glycosylation in Escherichia coli. Mol Microbiol. 2001;39:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 207. | Mironova R, Niwa T, Handzhiyski Y, Sredovska A, Ivanov I. Evidence for non-enzymatic glycosylation of Escherichia coli chromosomal DNA. Mol Microbiol. 2005;55:1801-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 208. | Boyanova M, Mironova R, Niwa T, Ivanov I. Post-translational Processing of Human Interferon-gamma and Approaches for its Prevention. Infectious Disease. Totowa: Humana Press 2008; 365-373. |
| 209. | Bozhinov AS, Boyanova M, Niwa T, Ivanov I, Mironova R. Evidence for the presence of glycation adducts in protein therapeutics. Biotechnol Biotechnol Eq. 2010;24:1904-1909. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 210. | Kleinova M, Belgacem O, Pock K, Rizzi A, Buchacher A, Allmaier G. Characterization of cysteinylation of pharmaceutical-grade human serum albumin by electrospray ionization mass spectrometry and low-energy collision-induced dissociation tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2965-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 211. | Bozhinov A, Handzhiyski Y, Genov K, Daskalovska V, Niwa T, Ivanov I, Mironova R. Advanced glycation end products contribute to the immunogenicity of IFN-β pharmaceuticals. J Allergy Clin Immunol. 2012;129:855-858.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 212. | Fernández O, Mayorga C, Luque G, Guerrero M, Guerrero R, Leyva L, León A, Blanca M. Study of binding and neutralising antibodies to interferon-beta in two groups of relapsing-remitting multiple sclerosis patients. J Neurol. 2001;248:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | Bertolotto A, Sala A, Malucchi S, Marnetto F, Caldano M, Di Sapio A, Capobianco M, Gilli F. Biological activity of interferon betas in patients with multiple sclerosis is affected by treatment regimen and neutralising antibodies. J Neurol Neurosurg Psychiatry. 2004;75:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 214. | Bendtzen K. Anti-IFN BAb and NAb antibodies: a minireview. Neurology. 2003;61:S6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 215. | Pachner AR, Oger J, Palace J. The measurement of antibodies binding to IFNbeta in MS patients treated with IFNbeta. Neurology. 2003;61:S18-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 216. | Pachner AR. An improved ELISA for screening for neutralizing anti-IFN-beta antibodies in MS patients. Neurology. 2003;61:1444-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 217. | Perini P, Calabrese M, Biasi G, Gallo P. The clinical impact of interferon beta antibodies in relapsing-remitting MS. J Neurol. 2004;251:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 218. | Borg FA, Isenberg DA. Syndromes and complications of interferon therapy. Curr Opin Rheumatol. 2007;19:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 219. | Marrie RA, Rudick RA. Drug Insight: interferon treatment in multiple sclerosis. Nat Clin Pract Neurol. 2006;2:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 220. | Gilli F, Marnetto F, Caldano M, Sala A, Malucchi S, Capobianco M, Bertolotto A. Biological markers of interferon-beta therapy: comparison among interferon-stimulated genes MxA, TRAIL and XAF-1. Mult Scler. 2006;12:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 221. | Kivisäkk P, Alm GV, Tian WZ, Matusevicius D, Fredrikson S, Link H. Neutralising and binding anti-interferon-beta-I b (IFN-beta-I b) antibodies during IFN-beta-I b treatment of multiple sclerosis. Mult Scler. 1997;3:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 222. | Rudick RA, Simonian NA, Alam JA, Campion M, Scaramucci JO, Jones W, Coats ME, Goodkin DE, Weinstock-Guttman B, Herndon RM. Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology. 1998;50:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 223. | Creeke PI, Farrell RA. Clinical testing for neutralizing antibodies to interferon-β in multiple sclerosis. Ther Adv Neurol Disord. 2013;6:3-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 224. | Grossberg SE, Oger J, Grossberg LD, Gehchan A, Klein JP. Frequency and magnitude of interferon β neutralizing antibodies in the evaluation of interferon β immunogenicity in patients with multiple sclerosis. J Interferon Cytokine Res. 2011;31:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 225. | Jensen PE, Sellebjerg F, Søndergaard HB, Sørensen PS. Correlation between anti-interferon-β binding and neutralizing antibodies in interferon-β-treated multiple sclerosis patients. Eur J Neurol. 2012;19:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 226. | Zare N, Zarkesh-Esfahani SH, Gharagozloo M, Shaygannejad V. Antibodies to interferon beta in patients with multiple sclerosis receiving CinnoVex, rebif, and betaferon. J Korean Med Sci. 2013;28:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 227. | Gibbs E, Karim ME, Oger J. Antibody dissociation rates are predictive of neutralizing antibody (NAb) course: a comparison of interferon beta-1b-treated Multiple Sclerosis (MS) patients with transient versus sustained NAbs. Clin Immunol. 2015;157:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 228. | Malucchi S, Sala A, Gilli F, Bottero R, Di Sapio A, Capobianco M, Bertolotto A. Neutralizing antibodies reduce the efficacy of betaIFN during treatment of multiple sclerosis. Neurology. 2004;62:2031-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 229. | Runkel L, Meier W, Pepinsky RB, Karpusas M, Whitty A, Kimball K, Brickelmaier M, Muldowney C, Jones W, Goelz SE. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta). Pharm Res. 1998;15:641-649. [PubMed] |
| 230. | Takahashi M, Fujii J, Teshima T, Suzuki K, Shiba T, Taniguchi N. Identity of a major 3-deoxyglucosone-reducing enzyme with aldehyde reductase in rat liver established by amino acid sequencing and cDNA expression. Gene. 1993;127:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 231. | Sato K, Inazu A, Yamaguchi S, Nakayama T, Deyashiki Y, Sawada H, Hara A. Monkey 3-deoxyglucosone reductase: tissue distribution and purification of three multiple forms of the kidney enzyme that are identical with dihydrodiol dehydrogenase, aldehyde reductase, and aldose reductase. Arch Biochem Biophys. 1993;307:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 232. | Feather MS, Flynn TG, Munro KA, Kubiseski TJ, Walton DJ. Catalysis of reduction of carbohydrate 2-oxoaldehydes (osones) by mammalian aldose reductase and aldehyde reductase. Biochim Biophys Acta. 1995;1244:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 233. | Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. J Clin Invest. 1998;101:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 390] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 234. | Collard F, Vertommen D, Fortpied J, Duester G, Van Schaftingen E. Identification of 3-deoxyglucosone dehydrogenase as aldehyde dehydrogenase 1A1 (retinaldehyde dehydrogenase 1). Biochimie. 2007;89:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 235. | Richarme G, Mihoub M, Dairou J, Bui LC, Leger T, Lamouri A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J Biol Chem. 2015;290:1885-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 236. | Van Schaftingen E, Collard F, Wiame E, Veiga-da-Cunha M. Enzymatic repair of Amadori products. Amino Acids. 2012;42:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 237. | Dimitrova AA, Stoilova , II , Atanasova , MA , Georgieva , MN , Russeva , AL , Strashimirov . Anti-Age antibodies and serum concentrations of zinc, copper and selenium in patients with occupational vegetative polyneuropathy of upper limbs. J Biomed Clin Res. 2009;2:31-35. |
| 238. | Fraser IP, Koziel H, Ezekowitz RA. The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin Immunol. 1998;10:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 148] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 239. | Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 812] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 240. | Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004;41:1099-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 241. | Mironova R, Sredovska A, Ivanov I, Niwa T. Maillard reaction products in the Escherichia coli-derived therapeutic protein interferon alfacon-1. Ann N Y Acad Sci. 2008;1126:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 242. | Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 243. | Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740-31749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 693] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 244. | Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting I. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 646] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 245. | Xue J, Ray R, Singer D, Böhme D, Burz DS, Rai V, Hoffmann R, Shekhtman A. The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry. 2014;53:3327-3335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 246. | Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987-14997. [PubMed] |
| 247. | Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, Bjercke RJ, Juhasz O, Crow MT, Tilton RG, Denner L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 256] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 248. | Odjakova M, Popova E, Merilin Al S, Mironova R. Plant-derived agents with antiglycation activity. Glycosylation. Rijeka: InTech-Open Access Publisher 2012; 223-256. [DOI] [Full Text] |
| 249. | Yuk IH, Zhang B, Yang Y, Dutina G, Leach KD, Vijayasankaran N, Shen AY, Andersen DC, Snedecor BR, Joly JC. Controlling glycation of recombinant antibody in fed-batch cell cultures. Biotechnol Bioeng. 2011;108:2600-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 250. | Makar TK, Nedergaard M, Preuss A, Hertz L, Cooper AJ. Glutamine transaminase K and omega-amidase activities in primary cultures of astrocytes and neurons and in embryonic chick forebrain: marked induction of brain glutamine transaminase K at time of hatching. J Neurochem. 1994;62:1983-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 167] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 251. | Susic D, Varagic J, Ahn J, Frohlich ED. Crosslink breakers: a new approach to cardiovascular therapy. Curr Opin Cardiol. 2004;19:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 252. | Coughlan MT, Forbes JM, Cooper ME. Role of the AGE crosslink breaker, alagebrium, as a renoprotective agent in diabetes. Kidney Int Suppl. 2007;S54-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |